Extracción de cataratas con facoemulsificación y lente intraocular de cámara posterior
Main Text
Table of Contents
La catarata es una de las principales causas de ceguera tratable en el mundo. Si bien existe una diferencia significativa en el acceso a la atención quirúrgica en los países en desarrollo frente a los países industrializados, la catarata es un contribuyente significativo a la discapacidad visual en ambos. El diagnóstico de cataratas se realiza mediante la evaluación de la agudeza visual, la discapacidad visual y la biomicroscopía con lámpara de hendidura. Las indicaciones comunes para la cirugía incluyen dificultad con el deslumbramiento, conducción nocturna, disminución de la mejor visión corregida, lo que afecta la distancia y / o la visión cercana, y deterioro de la visión de la retina que impide el tratamiento necesario. En los Estados Unidos, el estándar para la extracción de cataratas se ha convertido en facoemulsificación. El artículo demuestra y revisa la técnica de extracción de cataratas mediante facoemulsificación con implante de lente intraocular utilizando la técnica de divide y vencerás.
Los pacientes con cataratas visualmente significativas reportarán algún tipo de deterioro visual o deterioro funcional debido a una pérdida de agudeza visual. Esto puede ser tan simple como una disminución en la agudeza visual mejor corregida a distancia y / o cerca, o puede ser una pérdida más sutil de la función, como requerir más luz para leer, dificultad para conducir de noche, aumento del resplandor con los faros que se aproximan o resplandor solar al conducir cuando el sol está bajo en el horizonte. La información crítica aquí radica en documentar una disminución o pérdida del funcionamiento diario. Esta es la indicación para la intervención quirúrgica.
Agudeza visual
- Comprobar la agudeza visual del paciente con corrección actual.
- El estenopeico puede dar una referencia rápida sobre si la refracción puede ser beneficiosa o no.
- Refracción manifiesta para lograr la mejor agudeza visual corregida.
Pruebas de deslumbramiento
- La prueba de agudeza de brillo (BAT) se utiliza para demostrar el deslumbramiento.
- Esta prueba es útil para documentar la discapacidad visual con deslumbramiento en pacientes que tienen una buena agudeza visual mejor corregida.
Medidor de agudeza potencial (PAM)
- Esta es una prueba útil en pacientes con enfermedad ocular comórbida para ayudar a aconsejar a los pacientes con respecto al posible resultado de la visión después de la operación.
Hallazgos de la lámpara de hendidura
- Los párpados y los anexos deben examinarse para detectar cualquier signo de blefaritis, que debe tratarse antes de la operación. Esto también dará pistas sobre cualquier problema anatómico que pueda afectar la exposición quirúrgica.
- Se debe evaluar la conjuntiva. Esto puede afectar la posición de la incisión corneal, ya que puede haber cambios posquirúrgicos de la cirugía de glaucoma o pterigión que pueden limitar el acceso al limbo corneal.
- El examen de la córnea debe centrarse en la evidencia de cirugía corneal previa (cirugía refractiva o procedimientos de trasplante). Busque cicatrices u opacidades de cirugía, trauma o infección que puedan afectar la vista quirúrgica. Además, busque cualquier signo de distrofias que pueda afectar el resultado refractivo, así como predisponer a la descompensación endotelial después de la operación.
- La profundidad de la cámara anterior puede darle una idea de cuánto espacio tendrá para trabajar. Los ojos cortos con cámaras poco profundas tendrán facoemulsificación realizada un poco más posterior para limitar el daño endotelial.
- El iris debe evaluarse para la dilatación de la pupila, los defectos de transiluminación y el material de pseudoexfoliación. Estos son importantes para la evaluación de la exposición quirúrgica, así como la posible pérdida de estabilidad del complejo de lentes capsulares. Notar cualquier signo de trauma, compromiso zonular y / o mala dilatación ayudará en la planificación quirúrgica. El cirujano puede decidir tener dispositivos especializados disponibles para facilitar la cirugía, incluidos ganchos de iris, anillos de Malyugin, ganchos capsulares y anillos y segmentos de tensión capsular.
- La lente debe evaluarse para determinar la opacidad de la lente. También se debe evaluar la cápsula y el soporte zonular. Busque el centro del cristalino. Evalúe la estabilidad de la lente. El enfoque debe estar en cualquier signo de violación capsular o compromiso. Esto podría ser congénito, traumático o iatrogénico. Esto también es cierto para la pérdida zonular. La presencia de cualquiera de estos factores puede afectar el abordaje quirúrgico. En pacientes con interrupción significativa, la lensectomía pars plana puede ser el enfoque más seguro.
- Se debe realizar un examen dilatado. Esto ayudará a facilitar la evaluación de la lente. También es importante la evaluación de la retina y el nervio óptico. Esto ayudará a determinar si hay otras enfermedades que están afectando la visión, y si la cirugía de cataratas sería beneficiosa o si se puede necesitar otro tratamiento.
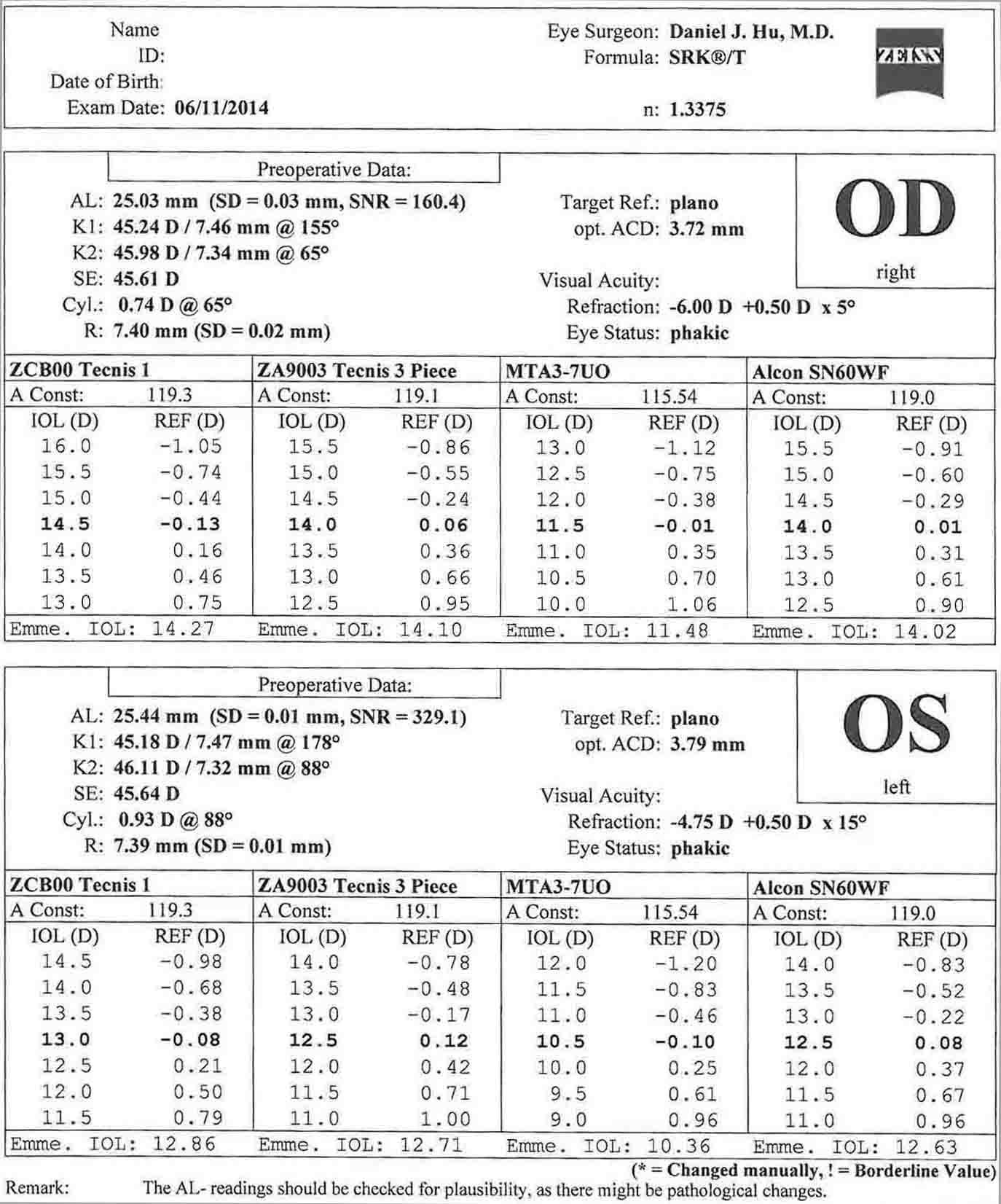
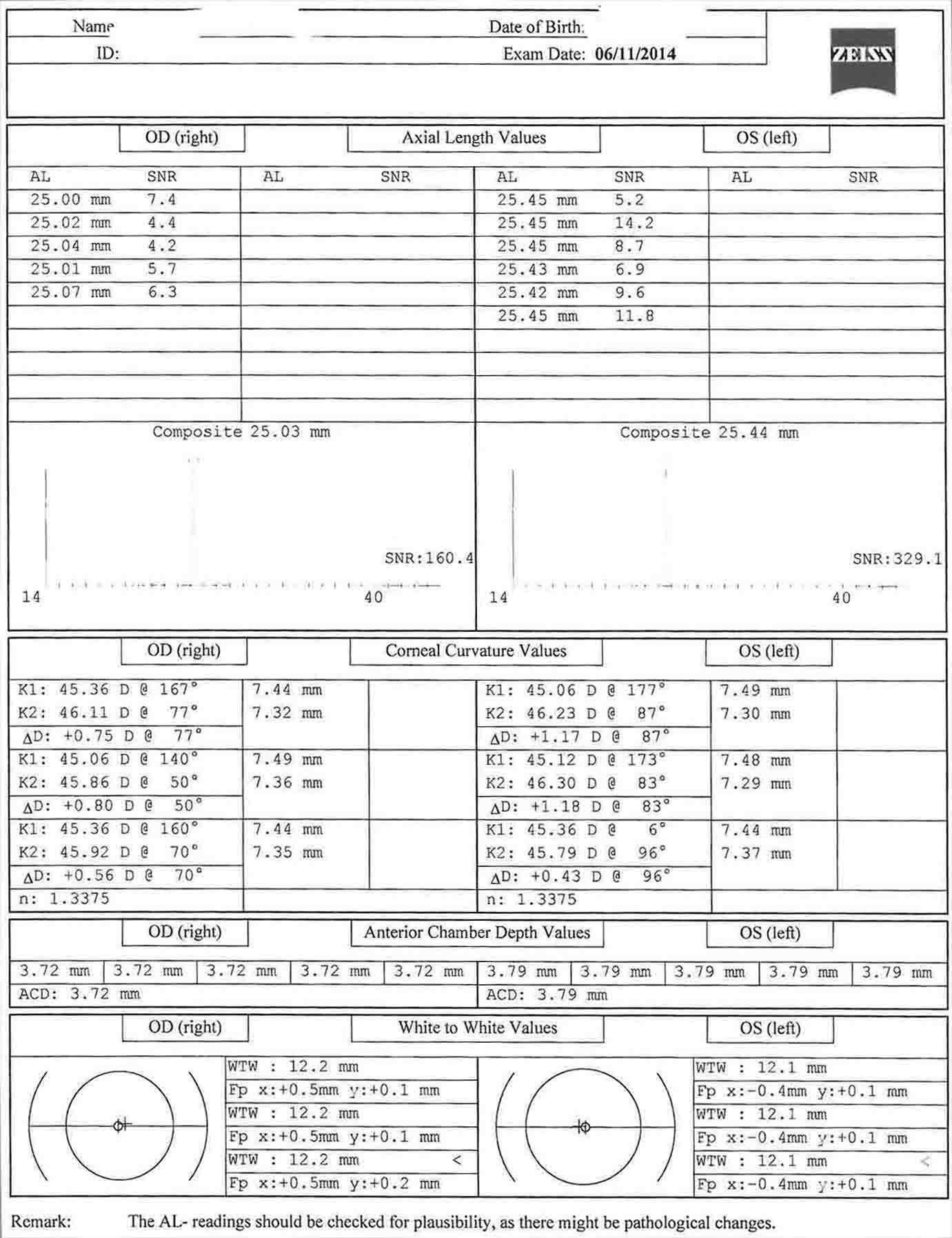
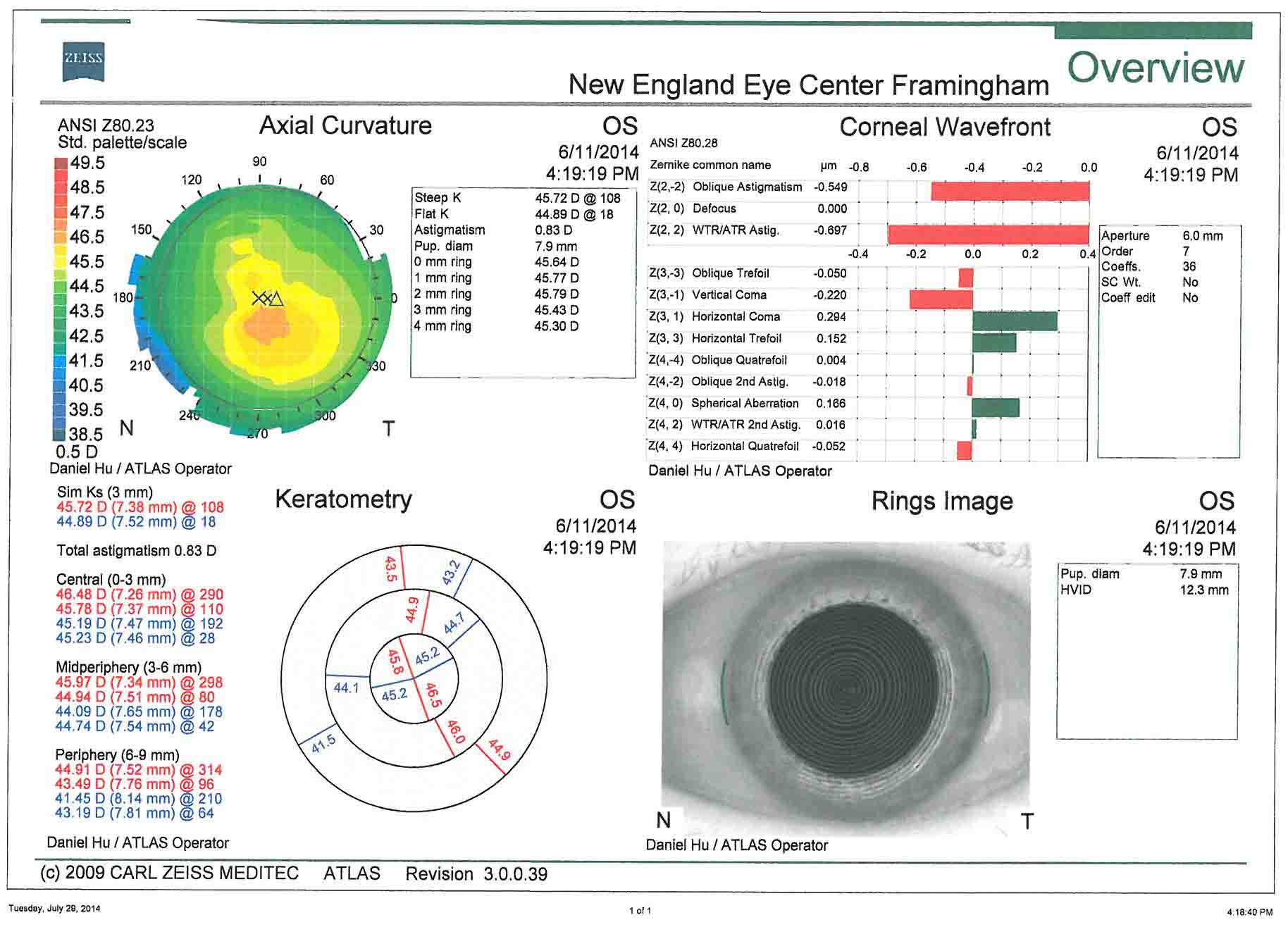
- Topografía corneal: es útil para la evaluación del astigmatismo corneal preoperatorio. La planificación quirúrgica del tratamiento del astigmatismo se basa en estas imágenes.
- Biometría (maestro de LIO): se mide la queratometría, la profundidad de la cámara anterior, la longitud de blanco a blanco y axial, y se calculan las potencias de la lente intraocular.
- B-scan- En casos de catarata madura que perjudica la visión de la retina. Esto permite una evaluación macroscópica de la anatomía de la retina. ¿Hay un desprendimiento de retina o masa presente?
- Microscopía especular- Se puede realizar en casos de disfunción endotelial para evaluar el endotelio antes de la cirugía.
En este caso, hay menos de 1 dioptría de astigmatismo corneal oblicuo en ambos ojos. Esto corrobora bien con la queratometría de la biometría. En este caso, las incisiones relajantes de la córnea podrían usarse para controlar el astigmatismo. El paciente ha optado por renunciar al tratamiento del astigmatismo.
- Si no se trata, la catarata conducirá a la pérdida progresiva de la visión y ceguera.
- Si la catarata es visualmente significativa, hay opciones limitadas para el tratamiento. La intervención quirúrgica es necesaria para restaurar la visión.
- La facoemulsificación mediante divide y vencerás tiene la ventaja de ser una técnica universal que se puede utilizar para todos los grados y tipos de cataratas.
- Se debe tener especial consideración en casos de esclerosis nuclear densa y madura, zonulopatía, subluxación del cristalino o cualquier caso con una visión limitada del segmento anterior.
Las posibles complicaciones postoperatorias incluyen las siguientes afecciones:
- Edema corneal
- Desprendimiento de membrana de Descemet
- Astigmatismo inducido
- Quemadura de la herida corneal
- Fuga de la herida
- Crecimiento epitelial descendente
- Síndrome del segmento anterior tóxico (TASS)
- Síndrome de iris flácido intraoperatorio (IFIS)
- Iridodiálisis
- Ciclodiálisis
- Síndrome de Urrets-Zavalia
- Presión intraocular elevada
- Glaucoma maligno
- Material de lente retenido
- Rotura capsular
- Prolapso vítreo
- Complicaciones de la LIO (descentración y luxación, captura pupilar, síndrome de bloqueo capsular, síndrome de uveítis-glaucoma-hipema, queratopatía bullosa pseudofáquica, potencia incorrecta de la LIO, deslumbramiento de la LIO, opacificación de la LIO)
- Fibrosis capsular anterior y fimosis
- Opacificación capsular posterior
- Hemorragia (hemorragia retrobulbar-complicación para anestesia retrobulbar, derrame supracoroideo, hemorragia supracoroidea expulsiva, hifema)
- Uveítis postoperatoria crónica
- Endoftalmitis
- Edema macular cistoide
- Toxicidad de la luz retiniana
- Infarto macular
- Desprendimiento de retina
La cirugía de cataratas ha experimentado un avance considerable en las últimas décadas que ha mejorado la seguridad y eficacia del procedimiento. Esto ha sido impulsado por un movimiento progresivo hacia la disminución del trauma quirúrgico en el ojo. La cirugía moderna de cataratas ha progresado desde técnicas de incisión grande, como la extracción de cataratas intracapsulares, hasta la extracción de cataratas extracapsulares, hasta la facoemulsificación de incisión pequeña. 1 La cirugía de cataratas se ha convertido en un procedimiento quirúrgico excepcionalmente seguro y exitoso. Múltiples estudios grandes han demostrado una mejor agudeza visual postoperatoria corregida de 20/40 o mejor en el 85,5-89% de todos los pacientes, y el 94,7-96% de los pacientes sin comorbilidades oculares preoperatorias. El 95% de los pacientes estaban satisfechos con el resultado de su cirugía. 2-4 Sin embargo, siguen existiendo desafíos en cuanto a la reproducibilidad de la capsulotomía, el uso del ultrasonido y sus efectos sobre el endotelio corneal, así como las complicaciones capsulares. A medida que se ha reducido el riesgo de complicaciones intraoperatorias significativas, la expectativa de resultados visuales ha seguido aumentando. La cirugía de cataratas y la cirugía refractiva están cada vez más vinculadas a medida que los resultados están siendo impulsados por la expectativa de emmetropía.
La cirugía de cataratas continúa evolucionando lejos de la cirugía manual a técnicas impulsadas por la tecnología. Un gran salto adelante fue el advenimiento de la facoemulsificación por Charles Kelman, MD en la década de 1960. En la década de 1990, la facoemulsificación se había convertido en el estándar para la cirugía de cataratas en los países desarrollados. Los refinamientos en la facoemulsificación han seguido mejorando los resultados y disminuyendo las complicaciones. La cirugía de cataratas asistida por láser de femtosegundo es la tecnología más nueva que se incorpora al uso con la cirugía de cataratas. Los beneficios potenciales de la cirugía de cataratas asistida por láser de femtosegundo incluyen capsulotomía reproducible, reducción del tiempo de ultrasonido e incisiones corneales reproducibles. Se ha demostrado una reducción significativa en el tiempo de ultrasonido. También se ha demostrado que los láseres de femtosegundo han mejorado la precisión y la reproducibilidad de la capsulotomía y las incisiones corneales. La reproducibilidad de las incisiones corneales es especialmente valiosa en el contexto del control del astigmatismo. 5-8 Los beneficios previstos de estas características son mejorar el tiempo de recuperación del paciente, reducir las complicaciones y ayudar a lograr mejores resultados refractivos.
A medida que las técnicas de cataratas han mejorado, también lo ha hecho la expectativa de una rápida recuperación postoperatoria, así como resultados refractivos inmediatos. La biometría mejorada ha aumentado la previsibilidad de los resultados de la cirugía poscatarata. La aberrometría intraoperatoria ha sido un gran activo para ayudar con la precisión de la selección de LIO, especialmente en ojos con cirugía queratorefractiva previa. 9 Esta previsibilidad es crítica cuando se consideran las expectativas refractivas tanto de los pacientes con cataratas de cirugía posrefractiva como de los pacientes con cirugía combinada de cataratas refractivas que esperan resultados inmediatos.
El ojo emétrope con adaptación para la presbicia se ha convertido en el objetivo de muchos pacientes. Si bien todavía hay espacio para que esta tecnología madure, la tecnología actual es capaz de lograr esto para muchos pacientes. El astigmatismo se puede controlar en el momento de la cirugía con cirugía en el eje, incisiones relajantes corneales o lentes intraoculares tóricas. 10-13 La presbicia se puede controlar con LIO monovisionales, multifocales o acomodativas. 14-15 Estas lentes intraoculares avanzadas han dado a los pacientes la capacidad de funcionar con mayor libertad del uso de la corrección de gafas para la mayoría de sus tareas diarias. Los avances en la tecnología de LIO continuarán impulsando el deseo de independencia del espectáculo después de la operación.
- Topógrafo corneal- Zeiss Atlas 9000
- Biometría- Zeiss IOL Master
- Facoemulsionante- Sistema de visión Alcon Infiniti
- Configuración
- Esculpir: Riego: 95 cm, Torsional: 100 (lineal), Vacío: 90 mmHg (lineal), Aspiración: 22 cc/min (fijo)
- Cuadrante: Riego: 100 cm, Torsional: 100 (lineal), Vacío: 350 mmHg (fijo), Aspiración: 40 cc/min (lineal)
- Epinúcleo: Irrigación: 95 cm, Torsional: 95 (lineal), Vacío: 300 mmHg (lineal), Aspiración 30 cc/min (lineal)
- Corteza: Irrigación: 100 cm, Vacío 500 mmHg (lineal), Aspiración: 35 cc/min (lineal)
- Pulido: Riego: 95 cm, Vacío 10 mmHg (lineal), Aspiración: 6 cc/min (lineal)
- Viscoelástico: Riego: 95 cm, Vacío 600 mmHg (Lineal), Aspiración: 40 cc/min (Fijo)
- Microscopio quirúrgico- Leica
- Lente intraocular- AMO Tecnis ZCB00 Lente intraocular
El autor no tiene relaciones financieras con ninguno de los productos o equipos mencionados en este artículo.
El paciente al que se hace referencia en este artículo de video ha dado su consentimiento informado para ser filmado y es consciente de que la información y las imágenes se publicarán en línea.
Citations
- Stein JD. Eventos adversos graves después de la cirugía de cataratas. Curr Opin Ophthalmol. 2012;23(3):219-225. doi:10.1097/ICU.0b013e3283524068.
- Lundström M, Barry P, Leite E, Stenevi U. 1998 European cataract outcome study: report from the European Cataract Outcome Study Group. J Catarata Refract Surg. 2001;27(8):1176-1184. doi:10.1016/S0886-3350(01)00772-6.
- Lum F, Schein O, Schachat AP, Abbott RL, Hoskins HD Jr, Steinberg EP. Inicial de dos años de experiencia con la base de datos de cirugía de cataratas de la Red Nacional de Resultados de Atención Ocular (NEON) de la AAO. Oftalmología. 2000;107(4):691-697. doi:10.1016/S0161-6420(99)00184-0.
- Jaycock P, Johnston RL, Taylor H, et al. The Cataract National Dataset electronic multi-centre audit of 55,567 operations: updating benchmark standards of care in the United Kingdom and international. Ojo (Lond). 2009;23(1):38-49. doi:10.1038/sj.eye.6703015.
- Reddy KP, Kandulla J, Auffarth GU. Efectividad y seguridad de la fragmentación del cristalino asistida por láser de femtosegundo y la capsulotomía anterior versus la técnica manual en la cirugía de cataratas. J Catarata Refract Surg. 2013;39(9):1297-1306. doi:10.1016/j.jcrs.2013.05.035.
- Abell RG, Kerr NM, Vote BJ. Hacia un tiempo de facoemulsificación efectivo cero utilizando el pretratamiento con láser de femtosegundo. Oftalmología. 2013;120(5):942-948. doi:10.1016/j.ophtha.2012.11.045.
- Hatch KM, Talamo JT. Cirugía de cataratas asistida por láser: beneficios y barreras. Curr Opin Ophthalmol. 2014;25(1):54-61. doi:10.1097/ICU.00000000000000013.
- Roberts TV, Lawless M, Chan CCK, et al. Cirugía de cataratas con láser de femtosegundo: tecnología y práctica clínica. Clin Exp Ophthalmol. 2013;41(2):180-186. doi:10.1111/j.1442-9071.2012.02851.x.
- Ianchulev T, Hoffer KJ, Yoo SH, et al. Biometría refractiva intraoperatoria para predecir el cálculo de la potencia de la lente intraocular después de una cirugía refractiva miópica previa. Oftalmología. 2014;121(1):56-60. doi:10.1016/j.ophtha.2013.08.041.
- Wang L, Misra M, Koch DD. Incisiones relajantes corneales periféricas combinadas con cirugía de cataratas. J Catarata Refract Surg. 2003;29(4):712-722. doi:10.1016/S0886-3350(02)01838-2.
- Kaufmann C, Peter J, Ooi K, et al. Incisiones relajantes limbares versus incisiones en el eje para reducir el astigmatismo corneal en el momento de la cirugía de cataratas. J Catarata Refract Surg. 2005;31(12):2261-2265. doi:10.1016/j.jcrs.2005.08.046.
- Sheppard AL, Wolffsohn JS, Bhatt U, et al. Resultados clínicos después de la implantación de una nueva LIO tórica acrílica hidrofóbica durante la cirugía de cataratas de rutina. J Catarata Refract Surg. 2013;39(1):41-47. doi:10.1016/j.jcrs.2012.08.055.
- Visser N, Bauer NJC, Nuijts RMMA. Lentes intraoculares tóricas: visión general, selección de pacientes, cálculo de LIO, técnicas quirúrgicas, resultados clínicos y complicaciones. J Catarata Refract Surg. 2013;39(4):624-637. doi:10.1016/j.jcrs.2013.02.020.
- Schmickler S, Bautista CP, Goes F, Shah S, Wolffsohn JS. Evaluación clínica de una lente intraocular difractiva asférica multifocal. Br J Ophthalmol. 2013;97(12):1560-1564. doi:10.1136/bjophthalmol-2013-304010.
- Cumming JS, Colvard DM, Dell SJ, et al. Evaluación clínica de la lente intraocular acomodadora Crystalens AT-45: resultados del ensayo clínico de la Administración de Alimentos y Medicamentos de los Estados Unidos. J Catarata Refract Surg. 2006;32(5):812-825. doi:10.1016/j.jcrs.2006.02.007.
- Gimbel HV. Divide y vencerás la facoemulsificación nucleofractis: desarrollo y variaciones. J Catarata Refract Surg. 1991;17(3):281-291. doi:10.1016/S0886-3350(13)80824-3.
Cite this article
Hu DJ. Extracción de cataratas con facoemulsificación y lente intraocular de cámara posterior. J Med Insight. 2023;2023(7). doi:10.24296/jomi/7.