Creation of a Radial-Cephalic Arteriovenous Fistula
,
Massachusetts General Hospital
Main Text
Table of Contents
End-stage renal disease is common in the United States. It is most commonly caused by diabetes and hypertension. Renal function progressively declines over an unpredictable period of months to years, such that the kidneys are no longer able to perform their function. If failing renal function is not corrected or aided, premature demise is certain. Fortunately, several reliable techniques exist for establishing durable vascular access to aid in renal replacement therapy, specifically hemodialysis. Here we present the case of a middle-aged male with progressive renal failure who underwent arteriovenous fistula creation for the purposes of aiding in renal replacement. We outline the scope of the problem, its natural history, preoperative care, selected intraoperative techniques, and relevant postoperative considerations for this process.
The world has seen an alarming rise in the number of patients with chronic kidney disease (CKD), with tens of millions of cases in the United States alone. In the United States, the easy availability of chronic hemodialysis (HD) as renal replacement therapy (RRT) enables it to serve as a safe and viable destination therapy for some, and as a bridge to transplant for others. Surgical hemodialysis access in the form of an arteriovenous fistula (AVF) provides the best conduit for HD.1
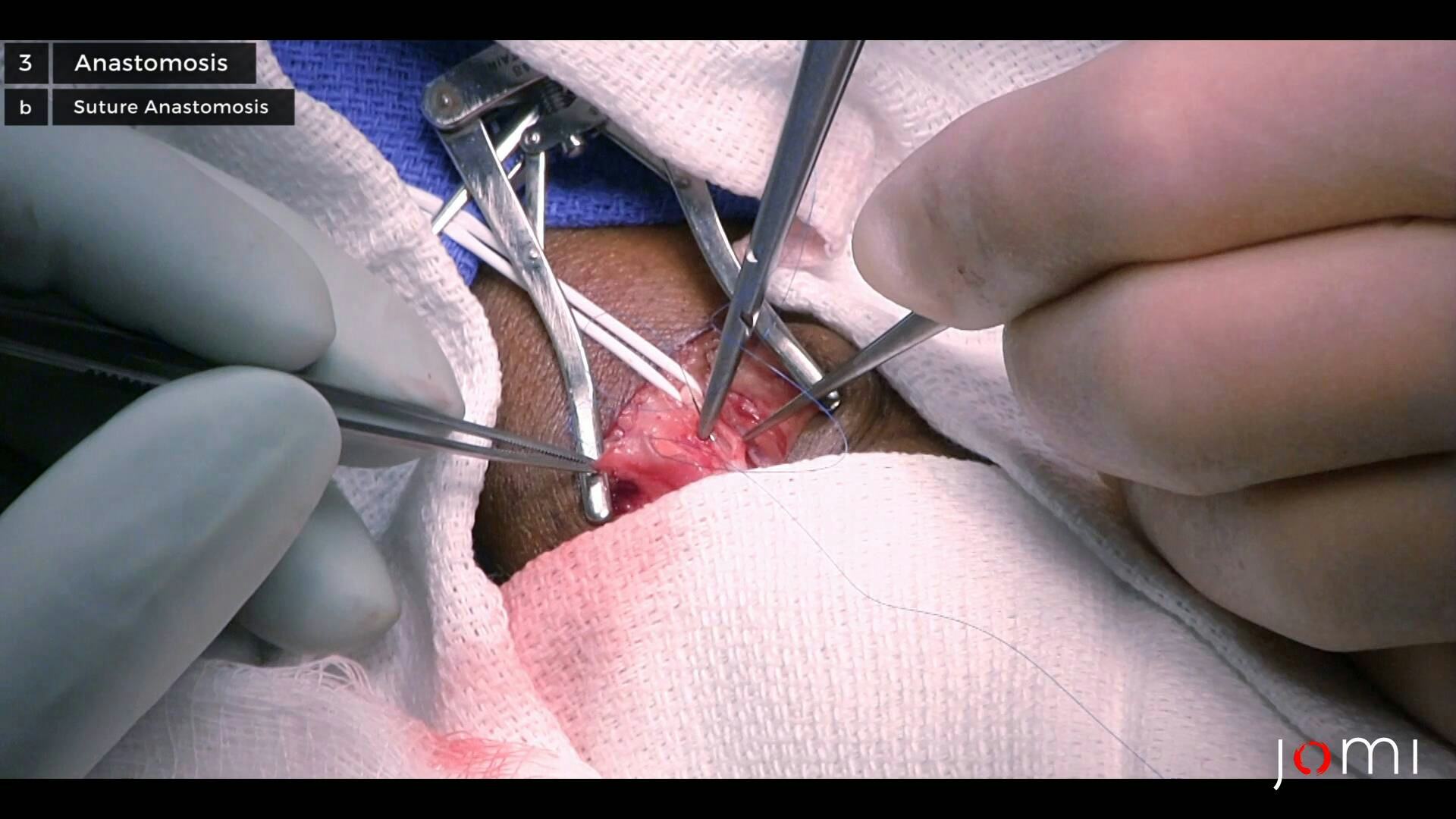
AVFs are surgically created connections between the native arteries and veins, allowing for reliable HD access capable of achieving the necessary blood flow rates for effective filtration. Here we will discuss the necessary steps in preoperative evaluation, as well as the surgical technique for creating a common type of AVF.
A thorough history and physical examination are part of the evaluation for AVF creation.
In particular, hand dominance and work history should be established. Evaluators should also assess for concomitant illnesses that may reduce the success of HD access creation.2, 3 Furthermore, a history of stroke, extremity incapacitation, chronic infections, skin diseases, and immunosuppression are important as these may affect the choice of procedure. Lastly, past surgical history should focus on access procedures, the venous system, and chest surgery.
Our patient is a 56-year-old right-hand dominant male with a history of obesity, type II diabetes, hypertension, hyperlipidemia, heart failure, and progressive renal failure. He has had no previous surgeries. Notable medications include aspirin, atorvastatin, isosorbide mononitrate, metoprolol succinate, and furosemide. Family history is notable for a sister with type II diabetes induced end-stage renal disease (ESRD) requiring renal transplantation. Our patient is retired, a social drinker, and a previous five pack-year smoker who quit 17 years prior.
Physical manifestations of chronic kidney disease (CKD) are often protean and may not appear until very late in the course of the disease. A comprehensive physical exam is important to assess suitability for surgery.
The preferred first site of fistula creation is the non-dominant arm. If this is not possible, the exam can be extrapolated to an alternate location. Examine for nearby edema, skin disease, scars, or extensive venous collaterals. Distal to the proposed site of AVF creation, the extremity should be free from neurovascular compromise.
Relevant arterial vasculature should be palpable, soft, easily compressible, and symmetric. Bilateral blood pressures should be obtained to ensure there are no obvious asymmetries, and Allen’s test should be performed to establish the presence of collateral flow distal to the proposed site of AVF creation. Alternatively, the Barbeau test has been noted to be more accurate and less subjective.4
In our case, the preoperative examination reveals an obese male. His heart rate is 51 beats per minute, and his left and right arm blood pressures are 139/66 mmHg and 152/72 mmHg, respectively. His heart is regular with a grade II/VI systolic murmur. His lungs have rales in the bases. He has 1+ pitting bilateral lower extremity edema, and his jugular venous pulsation is 2 cm above the clavicle when reclining at 45°. His left arm is free from skin abnormalities and demonstrates a normal Allen's test and a palpable cephalic vein in the forearm.
In preparation for AVF creation, ultrasound (US) mapping of the relevant arteries and veins is of the utmost importance. US imaging provides information about vein diameter, patency, distensibility, depth, congenital variations, and unsuspected pathology.1 Although it is operator dependent, the US has the advantage that it is non-invasive, evaluates both the arteries and veins in one setting, and does not require administration of nephrotoxic iodinated contrast. Moreover, it can be performed safely and quickly in the physician’s office and is easily repeated in the preoperative holding area.
If there are further questions concerning vascular disease, additional studies including contrast venography, arteriography, computed tomography, or magnetic resonance angiography are available as the adjunctive imaging techniques. These modalities must be utilized carefully given the toxic effects of various contrast media in the CKD patient.
The suggested minimum arterial diameter for successful AVF creation is 1.5–2 mm, but we prefer a minimum diameter of 2 mm.5, 6 With regard to venous anatomy, it is important that the lumen be ≥ 2.5 mm with the unobstructed flow. Additionally, it is ideal, though not imperative, that the selected segment be straight and < 1 cm from the skin surface. Many of these characteristics can be assessed by clinical examination, but the “2009 Kidney Disease: Improving Global Outcomes” guidelines recommend preoperative imaging.7
In our case, preoperative upper extremity vascular imaging is notable for a 3.8-mm cephalic vein and a 2.6-mm radial artery in the lower forearm. Both vessels are patent and without pathology.
Signs and symptoms of progressive renal dysfunction are insidious in onset and heterogeneous from patient to patient, and the determinants of progression are poorly understood.8 Factors such as HTN, diabetes, and proteinuria are known; however further investigation is ongoing to elucidate additional influences.9 As the disease progresses, patients will manifest symptoms of increasing morbidity and declining renal filtration, excretion, and endocrine function. It is known that CKD is strongly associated with poor outcomes, increased morbidity, and up to a 50% 5-year mortality rate.8
Patients with advanced CKD (a glomerular filtration rate of < 25 ml/min) should be referred to an access surgeon for evaluation for HD access.10 AV access should be created as soon as possible to allow proper “maturation” and to perform any additional procedures prior to HD initiation.1, 11 Evidence suggests that access construction 4–6 months prior to HD initiation is associated with fewer complications including death and sepsis.1, 11
There are three types of vascular access that are commonly utilized for performing HD12-15:
- Tunneled central venous catheters
- Arteriovenous grafts
- Arteriovenous fistulae
Autogenous arteriovenous access, created by anastomosing a native artery and vein to create a fistula, is the preferred method of HD access creation (AVF). This is due to its superior results in preventing failure and infection relative to other modalities.12-15
If the patient’s arterial or venous beds are not suitable for autogenous fistula creation, an arteriovenous fistula can be created with the aid of an interposed synthetic or biologic material (AVG). Its use is limited due to increased rates, relative to AVF, of both stenosis at the venous anastomosis, causing graft closure and of graft infection.12
Lastly, tunneled venous catheters provide immediate, short term HD access. However, they are fraught with acute and chronic complications such as thrombosis, infection, and central venous stenosis and generally should not be used in the long term.16
Of the commonly used methods, HD performed via autogenous AV access is superior with regard to patient morbidity and mortality with the possible exception in the elderly population (>75 years of age).13-15 Autogenous access is also associated with lower rates of patient death and infection and higher rates of graft patency.17 Additionally, initial AVF access should ideally be located as far distally in the upper extremity as possible in order to preserve more proximal locations for any future procedures.
In our case, the thorough preoperative evaluation revealed satisfactory arterial and venous conduits for the creation of a radio-cephalic AVF in the patient’s non-dominant forearm.
In preparation for AVF creation, it is important to ensure that the patient is healthy enough to undergo surgical intervention as this patient population can be quite ill at baseline.1 It is also important to ensure that there is no overlying skin or soft tissue infection at the proposed site.17
The preferred arterial conduit is the radial artery given its proximity to the cephalic vein relative to the ulnar artery.1 Furthermore, the cephalic vein is preferred to other veins given its ease of access and dissection. Lastly, the surgeon ideally utilizes standard surgical magnification while operating.
There is a growing burden of kidney disease worldwide. In the United States alone there are an estimated 20 million adults with CKD.10 The incidence of the disease has doubled since the beginning of the twenty-first century, growing most rapidly in adults older than sixty. The course of kidney disease is unpredictable with many sufferers progressing to ESRD and requiring dialysis. As of 2009, there were an estimated 871,000 people receiving treatment for ESRD.10
A patient with ESRD will require RRT in the form of hemodialysis, peritoneal dialysis, or renal transplant, to sustain life. The demand for donor kidneys is high; there are currently 100,791 people waiting for kidney transplants in the United States with 3,000 new patients being added to the waiting list each month. The median wait time is 3.6 years, and while awaiting transplantation, many patients become too ill to survive transplantation or pass away prior to being offered an allograft.18 Alternatively, there are 398,861 individuals in the United States receiving HD for their ESRD.10
In this article, we presented the case of a 56-year-old male with type II diabetes leading to ESRD. He underwent an uncomplicated left forearm radio-cephalic AVF creation and recovered without complication. He was seen in follow-up eight weeks after surgery and found to be in satisfactory health, but his GFR has continued to decline such that he is requiring increasing doses of furosemide to maintain a euvolemic state. On exam, the site of the AVF had a strongly-palpable thrill. A bruit was auscultated, but it had not yet matured to the point that could be accessed for HD. He will continue to meet with his outpatient nephrologist to assess the maturation of the fistula and plan for the eventual initiation of HD.
Over the past ten years, more than one million patients in the United States have initiated HD. This process has almost become routine and is now considered a “minor” procedure. This has been made possible through improved safety and refinement of vascular access creation techniques, and it is no small feat when considering the far-reaching effects of ESRD and that the patient population inherently has a diminished baseline secondary to comorbid disease.19
When chosen appropriately and monitored carefully, it is possible to safely perform HD access creation with a relatively low risk of complication. Of the available methods, AVF creation remains the most reliable method for providing RRT in patients with ESRD.12-15 However, outcomes studies of AVF creation note that primary failure of the fistula remains a major issue.20 There is no clear consensus on the predictors of this phenomenon, but it is thought that arterial diameter is most strongly associated with success or failure. Schinstock et al. found that the mean initial arterial diameters for patent brachiocephalic, brachiobasilic, and radiocephalic AVFs were 4.7 ± 1.0, 4.4 ± 1.2, and 2.9 ± 1.2 mm, respectively.20
When compared with other means of obtaining vascular access, AVFs present lower risks of morbidity in the form of infection and technical complications.19, 21 Additionally, the rate of cardiovascular and infection-related mortality after AVF creation is 3.1% versus 9.7% after catheter placement or 4.8% after graft placement.19 These estimates are amplified in the older and increasingly more comorbid patients. These morbidity and mortality risks are not trivial when considering the large number of people who will require RRT as a bridge or destination therapy.
With this burden in mind, multiple studies are underway to further refine the process of AVF creation and further optimize patient outcomes. It will be important to define testing modalities to ensure adequate extremity perfusion after fistula creation. In an effort to minimize burdensome complications, work is being done to refine alternative techniques of access creation such as a tunneled central venous catheter or AVG placement. Most importantly, work continues to better characterize biologic determinants of renal failure, as well as its progression towards ESRD requiring RRT.
We have nothing to disclose.
Consent for the use of clinical history, radiology, and the intraoperative video was obtained from the patient and providers involved in the compilation of this case report and filming.
Citations
- Sidawy AN, Spergel LM, Besarab A, et al. The Society for Vascular Surgery: clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. J Vasc Surg. 2008;48(5)(suppl):S2-S25. doi:10.1016/j.jvs.2008.08.042.
- Miller PE, Tolwani A, Luscy CP, et al. Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int. 1999;56(1):275-280. doi:10.1046/j.1523-1755.1999.00515.x.
- Astor BC, Coresh J, Powe NR, Eustace JA, Klag MJ. Relation between gender and vascular access complications in hemodialysis patients. Am J Kidney Dis. 2000;36(6):1126-1134. doi:10.1053/ajkd.2000.19816.
- Barbeau GR, Arsenault F, Dugas L, Simard S, Larivière MM. Evaluation of the ulnopalmar arterial arches with pulse oximetry and plethysmography: comparison with the Allen's test in 1010 patients. Am Heart J. 2004;147(3):489-493. doi:10.1016/j.ahj.2003.10.038.
- Malovrh M. Approach to patients with end-stage renal disease who need an arteriovenous fistula. Nephrol Dial Transplant. 2003;18(suppl 5):v50-v52. doi:10.1093/ndt/gfg1047.
- Silva MB Jr, Hobson RW II, Pappas PJ, et al. A strategy for increasing use of autogenous hemodialysis access procedures: impact of preoperative noninvasive evaluation. J Vasc Surg. 1998;27(2):302-308. doi:10.1016/S0741-5214(98)70360-X.
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;76(suppl 113):S1-S130. doi:10.1038/ki.2009.188.
- Jennings WC, Kindred MG, Broughan TA. Creating radiocephalic arteriovenous fistulas: technical and functional success. J Am Coll Surg. 2009;208(3):419-425. doi:10.1016/j.jamcollsurg.2008.11.015.
- Stringer S, Sharma P, Dutton M, et al. The natural history of, and risk factors for, progressive Chronic Kidney Disease (CKD): the Renal Impairment in Secondary care (RIISC) study; rationale and protocol. BMC Nephrol. 2013;14:95. doi:10.1186/1471-2369-14-95.
- Kidney disease statistics for the United States 2016. National Institute of Diabetes and Digestive and Kidney Diseases website. http://www.niddk.nih.gov/health-information/health-statistics/Pages/kidney-disease-statistics-united-states.aspx. Accessed August 1, 2017.
- Jindal K, Chan CT, Deziel C, et al; Canadian Society of Nephrology Committee for Clinical Practice Guidelines. Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology. J Am Soc Nephrol. 2006;17(3)(suppl 1):S1-S27. doi:10.1681/ASN.2005121372.
- Bachleda P, Utikal P, Kocher M, Cerna M, Fialova J, Kalinova L. Arteriovenous graft for hemodialysis, graft venous anastomosis closure - current state of knowledge. Minireview. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159(1):27-30. doi:10.5507/bp.2014.027.
- Onuigbo MAC. The natural history of chronic kidney disease revisited—a 72-month Mayo Health System Hypertension Clinic practice-based research network prospective report on end-stage renal disease and death rates in 100 high-risk chronic kidney disease patients: a call for circumspection. Adv Perit Dial. 2009;25:85-88. https://www.advancesinpd.com/adv09/306-Onuigbo-Final.pdf.
- Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int. 2001;60(4):1443-1451. doi:10.1046/j.1523-1755.2001.00947.x.
- Allon M, Daugirdas J, Depner TA, Greene T, Ornt D, Schwab SJ. Effect of change in vascular access on patient mortality in hemodialysis patients. Am J Kidney Dis. 2006;47(3):469-477. doi:10.1053/j.ajkd.2005.11.023.
- Vats HS. Complications of catheters: tunneled and nontunneled. Adv Chronic Kidney Dis. 2012;19(3):188-194. doi:10.1053/j.ackd.2012.04.004.
- Tordoir JHM, Bode AS, Peppelenbosch N, van der Sande FM, de Haan MW. Surgical or endovascular repair of thrombosed dialysis vascular access: is there any evidence? J Vasc Surg. 2009;50(4):953-956. doi:10.1016/j.jvs.2009.06.058.
- Organ donation and transplantation statistics 2017. National Kidney Foundation website. https://www.kidney.org/news/newsroom/factsheets/Organ-Donation-and-Transplantation-Stats. Accessed August 7, 2017.
- Stolic R. Most important chronic complications of arteriovenous fistulas for hemodialysis. Med Princ Pract. 2013;22(3):220-228. doi:10.1159/000343669.
- Schinstock CA, Albright RC, Williams AW, et al. Outcomes of arteriovenous fistula creation after the Fistula First Initiative. Clin J Am Soc Nephrol. 2011;6(8):1996-2002. doi:10.2215/CJN.11251210.
- Lok CE, Foley R. Vascular access morbidity and mortality: trends of the last decade. Clin J Am Soc Nephrol. 2013;8(7):1213-1219. doi:10.2215/CJN.01690213.
Cite this article
Elias N, Stapleton S. Creation of a radial-cephalic arteriovenous fistula. J Med Insight. 2020;2020(110). doi:10.24296/jomi/110.